Local
By By Mike Ralph Holyoke Enterprise
By By Mike Ralph Holyoke Enterprise
Sports
With six seniors and eight total letterwinners on the team, the Holyoke softball team has experience to build around.
By Diane Stamm | The Holyoke Enterprise
Six teams from Colorado, Kansas and Missouri competed in Holyoke this past week in the 2023 Midwest Plains regional 16-to-18-year-old Babe Ruth baseball tournament.
The summer competitive swim season has ended for the Holyoke Swim Team.
By Diane Stamm | The Holyoke Enterprise
Ag & Business
History Colorado is happy to announced the 25 Centennial Farms & Ranches honorees for 2023.
Sometimes finding hay is not so cut and dried. Thankfully, the 2023 Colorado Hay Directory is here for livestock owners looking for alfalfa, grass, mix or other hay products.
Recently in Kit Carson County, a producer contacted CSU Area Extension Livestock Specialist Travis Taylor with a mystery.
By Scott Stinnett | CSU Area Extension Specialist
School
The Melissa Memorial Hospital Foundation has awarded four new scholarships and renewed two scholarships to previous recipients for the 2023-2024 school year.
Josephine Schlachter from Holyoke was named to the University of Sioux Falls Spring 2023 Dean’s List. Schlachter is majoring in biology.
Editor's Picks
-

,Winners of PCED’s microgrant awards are Aly Lock, Kaden Herman, Grace Gibson, Carter Van Overbeke, Caden Sporhase, Jaxson Hutches, Kelsie Hadeen, Kinsley Koberstein, Ethan Schneller, Tayla Martin and Teagan Martin.

,Winners of the group awards are Jimena Nuñez, Hannah Lindholm and Fatima Nuñez for the Holyoke Shop Local campaign; and Michael Gerk, Kyle Fryrear and Maclin Tempel for Haxtun’s Small Business Showcase.

Winners of the individual awards are Ryan Davis for his business, “The Shirt Off My Back,” and Christopher Durbin for his business, “Duo Farms.”
-

,Hodgson (Knobbe) brothers Dave, at left, and Bill, are shown with a photo of their father, and engraved plane wreckage gifted to them by Loren and Colette Jessen. — Shari Friedel | High Plains News North

,A photo taken by then-land owner George Steudler shows the cleanup effort by Air National Guard. — Shari Friedel | High Plains News North

Engraving is visible on one end of the wrecked airplane part found in Perkins County, Nebraska. — Shari Friedel | High Plains News North
-

,Pictured with their FBLA state conference plaques are, from left, Fatima Nuñez, Hannah Lindholm and Jimena Nuñez, second place in Partnership with Business; Lucinda Mares, second place in Introduction to Parli Pro; and Carter Van Overbeke, Caden Sporhase and Jaxson Hutches, second place in American Enterprise Project. — Courtesy Photo

Holyoke FBLA members gather for a photo at the state conference at the Gaylord Hotel in Aurora in April. — Courtesy Photo
-

,— Jerel Domer | The Holyoke Enterprise

,— Jerel Domer | The Holyoke Enterprise

,Holyoke Elementary students emerge from the school on the last day of class, May 25, by all appearances ready for a well-earned summer break. — Jerel Domer | The Holyoke Enterprise

,— Jerel Domer | The Holyoke Enterprise

Holyoke’s sixth graders took on the school staff in a kickball game on the last day of school, May 25. Pictured at top, Lili Vasquez takes her shot against teachers including, from left, Matt Ramirez, Kaylee Duvall, Jesus Trejo, Stefan Betley, Jordan Gerk and Yesenia Bencomo. In the bottom photo, Yesenia Bencomo hits one high, while Ricardo Moreno, Raia Sprague and Mason McCormick prepare to attempt a catch. — Jerel Domer | The Holyoke Enterprise
-
-

,Just beating the ball thrown by the Las Animas pitcher, Caiden Krueger dives safely back to first base. — Photo by Jes-c French

While the Crowley County infield waits for the ball to be thrown back in, Cash Weber rounds third base, sent home on a double hit by teammate Tyson Mosenteen. — Photo by Jes-c French
-
-

,Educators, family and friends cheer as graduates throw their caps in the air, marking a milestone achievement for the Holyoke High School Class of 2023. — Jerel Domer | The Holyoke Enterprise

,Itzel Apodaca-Saenz, pictured at right, shakes hands with school board secretary Jessica Koch as she receives her diploma. — Jerel Domer | The Holyoke Enterprise

,Angel Carrasco grins ear to ear as he makes his way to the stage during the graduation processional. — Jerel Domer | The Holyoke Enterprise

Capless HHS grads set forth at the end of the graduation ceremony with diplomas in hand. — Jerel Domer | The Holyoke Enterprise
-

,Pictured above, Jazlynn Leyva hugs Dragon’s Wagon Preschool director Marcia Walter while holding her diploma at the Dragon’s Wagon ceremony on Thursday, May 11, at Phillips County Event Center. Among the many songs sung with gusto by the graduating preschool class were “Rainbow Sharks,” “Colors All Around Us” and “I Can Paint a Rainbow.” — Jerel Domer | The Holyoke Enterprise

Pictured above, Dragon’s Wagon preschoolers take the stage to sing songs for friends and family for their end-of year program. — Jerel Domer | The Holyoke Enterprise
-

,The Queen of Spades balances swords upon each other the evening of Sunday, May 7, at Circus Wonderland in Holyoke. Performers wowed audiences with “Alice and Wonderland”-themed feats involving balancing, illusions and spinning objects. — Photo by Elly Brown

A clown, right, performs with a dad chosen from the audience. By combining four dads, the clown formed a tabletop. — Photo by Elly Brown
-

,Alma Alejandre receives a certificate of recognition the evening of Thursday, April 27, at the Awards and Officer Installation Ceremony for the Holyoke chapter of Future Business Leaders of America. This year, 61 students participated in FBLA. — Jerel Domer | The Holyoke Enterprise

New Future Business Leaders of America line up following the Awards and Officer Installation Ceremony. Pictured from the left are Secretary Hannah Lindholm, Treasurer Elyce Sisseck, Social Media Representative Katelyn Kropp, President Tayla Martin, Vice President Carter Van Overbeke, Fundraising Coordinator Ben Kleve and Information Officer Cozner Ring. — Jerel Domer | The Holyoke Enterprise
-
-

,General Manager Dennis Herman speaks to members of the Highline Electric Association energy cooperative the afternoon of Tuesday, March 28, at the Phillips County Event Center. — Andrew Turck | The Holyoke Enterprise

Merlin R. Prior, secretary for District 4 of Highline Electric, bring a Hamilton Beach blender to a door-prize winner late into the cooperative’s annual meeting. — Andrew Turck | The Holyoke Enterprise
-

,Sew Blessed member Teri Wernsman holds up one of the sewing machine bags used to hold supplies for the machine pictured. It matches the machine’s cover. — Courtesy Photo

Sew Blessed founder Maureen Waite stands in front of one of the cabinets in the children’s play room used to store items set to be donated. She is holding up a pillow that can be used by nursing home residents to place in back of their neck. — Courtesy Photo
-

,Happy Jacks Barbeque owners Sheila and Allyn Robinson, from the left, are pictured in their restaurant beside Karla and Jose Varela, who are set to become the new owners by Thursday, March 2. Karla has been working as a cook for Happy Jacks since its former identity as R&B Catering. — Andrew Turck | The Holyoke Enterprise

Brittany Daniel avoids a dollop of gravy on Monday, Jan. 23, during the Holyoke Chamber of Commerce’s 31st Annual Gala. Happy Jacks, who catered the event, won the chamber’s STAR Award for 2022. — Andrew Turck | The Holyoke Enterprise
-

,A Ford Police Interceptor belonging to Thomas Elliott, the late Phillips County sheriff, sits outside the County Courthouse Tuesday afternoon. People began to decorate the vehicle soon after it was parked. — Andrew Turck | The Holyoke Enterprise

Attendees line the pews of St. Patrick’s Catholic Church during the Monday morning funeral for Phillips County Sheriff Thomas Elliott. Following the mass, he was interred at Holyoke Cemetery. — Frank Perea II | The Holyoke Enterprise
-

,On Feb. 14, Regent Park and Carriage House celebrated Valentine’s Day with a party. The party included dancing, crowning of kings and queens, and a variety of heart-shaped brownie bites, appetizers and community. Crowned kings and queens are pictured from left, Harold Hadeen, Vickie Hadeen, Margie Jeffers and Kenneth Hansen. — Photo Courtesy of MegAnn V. Mari

,Pictured from left are Dixie Stuart, Raymond Gerk and occupational therapist Hannah Eckert. — Photo Courtesy of MegAnn V. Mari

Pictured above, Lois Schlachter and Sherman Kage are dancing. — Photo Courtesy of MegAnn V. Mari
-

,Hudson Koellner of Holyoke, wrestling heavyweight, wraps up Burlington’s Diego Estrada during his first match of the Class 2A Region 1 tournament on Friday in Sterling, Colo. He won by fall at 2:38. — Andrew Turck | The Holyoke Enterprise

Tyson Mosenteen of Holyoke maintains control during his 157-pound match Saturday against Angel Vela of Burlington. He took the bout 8-2 in what Holyoke Head Coach Broc Pelle called “a very smart, technical match.” — Andrew Turck | The Holyoke Enterprise
-

,This more than 4-foot-long, 9,000-piece Lego replica of the Titanic – constructed lights and all by Holyoke resident Tyler Weiss – served as a main attraction Saturday night at Peerless Theatre’s CineMagic fundraiser, themed around the doomed vessel. While the total amount raised was unavailable at press time, the event’s live and silent auctions reportedly netted organizers more than $6,800. — Andrew Turck | The Holyoke Enterprise

,Paula Strode laughs during the CineMagic fundraiser after Lloyd Michael of Michael Auction Service tricks her into raising her hand to bid on an item. Strode serves on the Golden Plains Recreation/Peerless Center Board of Directors, along with its President Rich Hielscher, Vice President Jerry Rohr, Secretary Lillian Garcia, Treasurer Chris Mattson and fellow member Thom Elliott. — Andrew Turck | The Holyoke Enterprise

Tricia Michael struggles to keep a cooler raised as auctioneer Lloyd Michael expounds upon the item’s many virtues during the CineMagic fundraiser live auction. Event supporters included 19 table and ticket sponsors, eight monetary donors, 21 silent auction donors and 10 live auction donors. Following the auction, participants hit the poker and craps tables, with Gary Fiscus, Tom Gertner and Ron Miles acting as dealers. — Andrew Turck | The Holyoke Enterprise
-

,Fire continues to smolder Thursday afternoon within the Nutrien Ag Solutions facility. Firefighters used limited water at the facility’s burnout areas to avoid runoff from hazardous chemicals. — Emmy Brown | The Holyoke Enterprise

Smoke escapes and billows upwards last Wednesday at an early afternoon fire at Nutrien Ag Solutions, located along Highway 6 in Lamar, Nebraska. — Diane Stamm | The Imperial Republican
-

,In the battle for a loose ball, the Holyoke Dragons’ Hannah Lindholm grabs the ball from between Haxtun player Anessa Colglazier’s ankles. — Diane Stamm | Holyoke Enterprise

Hannah Lindholm of the Holyoke Dragons, right, defends against a pass by Haxtun’s Grace Gibson. The Dragons put up a fight against Haxtun’s Bulldogs, despite missing two seniors, but eventually fell 37-50. — Diane Stamm | For The Holyoke Enterprise
-

,A water drop falls in Holyoke from an icicle upon Hometown Liquors’ awning the afternoon of Wednesday, Jan. 4. Data compiled by Dan Kafka of the National Weather Service’s Cooperative Observer Program indicate Phillips County received just over half of its average 18 inches in precipitation. — Andrew Turck | The Holyoke Enterprise

This graph shows annual precipitation for Phillips County recorded by Dan Kafka for the past decade. Before 2022, the county hit another low in 2012 with 10.34 inches. — Andrew Turck | The Holyoke Enterprise
-

,With a corn field as a backdrop on July 25, Holyoke Community Childcare Initiative program coordinator Trisha Herman explains what a proposed child care center could be like on this piece of land at the southeast edge of Holyoke. — Darci Rodriguez | The Holyoke Enterprise

,Anna Beth Love, a granddaughter of Holyoke’s late Mayor Orville Tonsing, approaches Tonsing’s casket on Monday, Oct. 17, during his celebration of life Monday morning. Tonsing is survived by two children, two grandchildren, four great-grandchildren and two great-great-grandchildren. — Andrew Turck | The Holyoke Enterprise

Michael Hassell, right, the new CEO for Melissa Memorial Hospital, chats with lab technician Stephanie McKenzie late the afternoon of Tuesday, Nov. 29. He began work at the hospital following his Oct. 18 appointment by its Board of Directors. — Andrew Turck | THe Holyoke Enterprise
-

,The trophy presentation at Budweiser Events Center in Loveland on Saturday, March 12, was cause for celebration for the Holyoke Lady Dragons, who won their first ever state title. Players are pictured from left, Leah Struckmeyer, Elyce Talavera, Grace Roberts, Hannah Lindholm, Audrey Talavera, Ava Koberstein and Kristin Vieselmeyer. — Darci Rodriguez | The Holyoke Enterprise

,In her home in Holyoke, Maria del Rosario Olivas, aka Chayo, shows how to make corn tamales, which use hand-ground corn masa and corn husks. She is also well known
for her traditional corn tortillas. — Darci Rodriguez | The Holyoke Enterprise
Jessie Owens competes in the sheep show at Phillips County Fair. The Holyoke High School sophomore died in a one-car accident March 21. She will be remembered for her love of animals, children, senior citizens, sports, welding and more. — Courtesy Photo
-

,Michael Hassell, right, the new CEO for Melissa Memorial Hospital, chats with lab technician Stephanie McKenzie last Tuesday afternoon. He began work at the hospital following his Oct. 18 appointment by its Board of Directors. — Andrew Turck | The Holyoke Enterprise

Michael Hassell takes a selfie with his co-workers during a Melissa Memorial Hospital appreciation lunch. Pictured clockwise from Hassell are Chief Compliance Officer Jimmie Bailey III, Operating Room Manager Elly Bailey, Registered Nurse Amanda Austin, Admissions Workers Brylea and Carrie Waln, and Board President Steve Young. — Courtesy Photo
-

,The house at 126 West 12th Street in Imperial, Nebraska, was the scene of a shooting Thanksgiving night close to midnight. Nineteen-year-old Jesse Krausnick died from his injuries after the incident. — Jan Schultz | The Imperial Republican

Jesse Krausnick is pictured at left, Tristan Ferguson is pictured at right. — Courtesy Photo
-

,There may not be much the characters played by Paul Brunner, Chase Oman and Penny Dockins can agree on, but one thing is certain: It’s always a good idea to feed the actors first. — Photo by Jes-c French

Playing a firefighter-cum-actor, Eric Conklin definitively answers the question of whether anyone in the troupe can dance. — Photo by Jes-c French
-

,Max Kleve of the Holyoke junior varsity team sends a shot soaring toward the basket Dec. 2 in a game against the Lone Star Longhorns. Despite their tough defense, the Holyoke Dragons lost 36-60. — Photo by Elly Brown | For The Holyoke Enterprise

,Members of the Holyoke High School girls basketball team are pictured from left, front row, Sophia Bencomo, manager Flor Alejandre, manager Maelynn Frost, manager Emersyn Goldenstein, Valeria Morales and Alma Alejandre; second row, Audrey Talavera, Elyce Sisseck, head coach Arlan Scholl, assistant coach Chandler Gerk, Hannah Lindholm and Leslie Carrasco; and back row, Nicole Schlachter, Elise Krogmeier, Annessa Colglazier, Ellie Kleve, Grace Roberts, Leah Struckmeyer and Jersie Gipson. Not pictured is Annette Moreno. — Photo by Jim Powell Photography

Members of the Holyoke High School boys basketball team are pictured from left, front row, Cozner Deaver Ring, Isaac Cummings, Ben Austin, Gustavo Goytia, Adrian Torres, Omar Hernandez, Mason Sprague and Edwin Perez; second row, Teagan Martin, Edel Ramos, Ivan Valenzuala, assistant coach Marcus Kammer, head coach Colbey Stumpf, Mason Powell, Cash Weber and Colby Weber; third row, Brendan Nelson, Edgardo Moncada, Irving Dominguez, Manuel Gonzalez, Cooper Goldenstein, Nik Rascon and Caiden Krueger; and back row, Reid Sprague, Max Kleve, Kai Siep, Luke Roberts, Wyatt Sprague and Chase Johnson. Not pictured are managers Dathyn Dirks and Cruse Weber. — Photo by Jim Powell Photography
-

,Isaiah Rueter, as Shrek, wards off fairy tale creatures fawning over him after his reluctant offer of assistance. Reuter has performed in multiple productions including “Clue,” “Damn Yankees,” “The Addams Family” and “Much Ado About Nothing.” — Andrew Turck

,Kyrah McConachie, as the Fairy Godmother, gets into position during the “Story of My Life” musical number. For the high school senior, this production was her first time acting in a full musical. — Andrew Turck

,Hailey Clapper, as Pinocchio, laments the state of life after being kicked out of Lord Farquaad’s kingdom and into a swamp. Before “Shrek,” Clapper acted in “Singing in the Rain.” — Andrew Turck

Fairy tale creatures reach a crescendo during the song “Story of My Life” near the play’s beginning. At the top of the rabble stands high school senior Daniela Fierro as the Wicked Witch. Fierro wrote in the program that she hopes to continue theatre in college. — Andrew Turck
-

,Monserrato Conde III holds a photo of himself taken when he joined the Army National Guard. As a member of the 140th Signal Battalion in Sterling from 1989-98, he helped maintain a communication network through the city. — Andrew Turck | The Holyoke Enterprise

,Matt Meusborn of Holyoke helps resident Gary Herr cut a piece of door trim at his residence Saturday afternoon. Meusborn cites his work with the U.S. Army’s 1st Battalion, 92nd Field Artillery as helping him learn the importance of working well with others. — Andrew Turck | The Holyoke Enterprise

Matt Meusborn’s scrapbook shows displays scenes from his time in the 1st Battalion, 92nd Field Artillery unit. — Andrew Turck | The Holyoke Enterprise
-

,Monserrato Conde III holds a photo of himself taken when he joined the Army National Guard. As a member of the 140th Signal Battalion in Sterling from 1989-98, he helped maintain a communication network through the city. — Andrew Turck | The Holyoke Enterprise

,Matt Meusborn of Holyoke helps resident Gary Herr cut a piece of door trim at his residence Saturday afternoon. Meusborn cites his work with the U.S. Army’s 1st Battalion, 92nd Field Artillery as helping him learn the importance of working well with others. — Andrew Turck | The Holyoke Enterprise

Matt Meusborn’s scrapbook shows displays scenes from his time in the 1st Battalion, 92nd Field Artillery unit. — Andrew Turck | The Holyoke Enterprise
-

,Holyoke’s Reid Sprague catches the football with two Monte Vista defenders covering during Saturday’s playoff game in Monte Vista. — Brian Williams | Monte Vista Journal

,Holyoke’s Wyatt Sprague (2) passes during the first half of Saturday’s playoffs loss, 28-21, to Monte Vista. — Brian Williams | Monte Vista Journal

Holyoke’s Tyson Mosenteen runs for a touchdown in the fourth quarter to close the gap to seven points in Saturday’s playoff football game against Monte Vista on Saturday. — Brian Williams | Monte Vista Journal
-

,Owen Ortner watches a camera lens intently while dressed as a Minion. He won $5 at the Holyoke Lions Club’s Halloween costume parade in the age 3 category.

,Jasely Leyva, dressed as Frida Kahlo, expresses delight from First Pioneer National Bank’s candy stand as costumed residents pass by.

,Kingsley Rowan pokes her head out between stuffed cats made in her likeness Monday evening to discern her surroundings. Later that day, she would take second in the Lions Club costume contest under the category of children age 2.

,Christian Loya gets his hat adjusted while “riding” an inflatable tyrannosaur toward Trunk or Treat festivities in Holyoke. Dinosaur costumes were a regular occurrence both there and in Trick or Treat the Town along Interocean Avenue.

Jackie Hale defends against a sudden onrush by a man dressed as a giant pig. No animals were harmed that evening during Melissa Memorial Hospital’s annual Trunk or Treat event, though one goat was dressed up as a unicorn.
-

,Holyoke players celebrate as Burlington players pick themselves off the floor following the final point of Holyoke’s five-set win over the Cougars Friday evening. — Diane Stamm | The Holyoke Enterprise

Erin Andersen tips a pass over the net against Burlington. — Diane Stamm | The Holyoke Enterprise
-

,Holyoke sixth grader Kyle Goldenstein sits in the driver's seat of a JDX Racing vehicle. Her father Luke Goldenstein hosted an event Oct. 13 to teach local students about opportunities in the racing industry. — Courtesy photo

,Luke Goldenstein shows students a vehicle's steering wheel. — Courtesy photo

Students take a look under the hood of a JDX racing car. All students in the district were welcome to attend. — Courtesy photo
-

,Anna Beth Love, a granddaughter of Holyoke’s late Mayor Orville Tonsing, approaches Tonsing’s casket during his celebration of life Monday morning. Tonsing is survived by two children, two grandchildren, four great-grandchildren and two great-great-grandchildren. — Andrew Turck | The Holyoke Enterprise

Volunteer firefighter Bob Heldenbrand (second from the left) watches as Orville Tonsing’s casket is lowered beneath Holyoke Cemetery. — Andrew Turck | The Holyoke Enterprise
-

,Sarah Rueter, pictured in the center, waves to the crowd from the Dragon’s Wagon Preschool float in the Holyoke High homecoming parade on Thursday, Oct. 6. See Page 8 for more photos from the homecoming festivities. — Photo by Jes-c French

,Alexis Nelson, at right, plays with the band at the homecoming pep rally following the parade on Thursday. — Photo by Jes-c French

,Sophomores Brandon Talich and Parker Steggs, from left, have some fun during the homecoming Olympic games on Thursday morning last week. — Photo by Jes-c French

Freshmen Anessa Colglazier and Belle Kropp compete in the wheelbarrow race during homecoming Olympics last week. — Photo by Jes-c French
-

,With red lipstick to match her pretty red sweater, Ruby Trujillo-Cordova celebrates her 100th birthday last weekend. This fashionable, music-loving great-great-great-grandmother is a resident at Regent Park nursing home in Holyoke. — Courtesy photo

,This photo of Ruby Trujillo-Cordova gives people a sneak peek of what’s inside a CD case — a special recording of her own music. Her family and friends remember how much she loved singing and playing guitar. — Courtesy photo

,Jaslene Dominguez, at right, a Holyoke fourth grader, celebrates with her 100-year-old great-great-grandma Ruby Trujillo-Cordova on Monday, Sept. 19, at Regent Park. — Darci Rodriguez | The Holyoke Enterprise

Centenarian Ruby Trujillo-Cordova keeps her wedding photo in her bedside table at Regent Park nursing home. — Courtesy photo
-

,Dance instructor Alma Nydia Nuñez Montes mesmerizes the audience with a dance called El Polvorete that represents the Mexican state of Jalisco. — Darci Rodriguez | The Holyoke Enterprise

,Junior high and high school dancers perform Las Alazanas/La Negra, which highlights the culture of the Mexican state of Jalisco. Pictured from left are Alma Alejandre, Michell Trejo, Fatima Castillo, Andrea Marquez, Vianey Jimenez and Vanessa Zapata. The folkloric dance recital was part of Phillips County Family Education Services’ 30th anniversary celebration Aug. 3 at the JR/SR high school auditorium. — Darci Rodriguez | The Holyoke Enterprise

,Valentina Gonzalez dances to Sones de Ixtapa, a dance from Chiapas, Mexico. — Darci Rodriguez | The Holyoke Enterprise

,Phillips County Family Education Services executive director Linda Jelden, at right, and Cecilia Marquez share about the 30-year history of the nonprofit. — Darci Rodriguez | The Holyoke Enterprise

Husband and wife dance instructors Alma Nydia Nuñez Montes and Ivan Olivas Castillo perform a folkloric dance from Chihuahua, Mexico. — Darci Rodriguez | The Holyoke Enterprise
-

,With a corn field as a backdrop, Holyoke Community Childcare Initiative program coordinator Trisha Herman explains what a proposed child care center could be like on this piece of land on the southeast edge of Holyoke. — Darci Rodriguez | The Holyoke Enterprise

,Attendees at a July 25 campaign kickoff event gather around Trisha Herman, Holyoke Community Childcare Initiative program coordinator, on a piece of land southwest of First Baptist Church. HCCI hopes to raise $1 million for a $3.35 million child care facility in hopes of breaking ground here by 2023. The initiative has already raised $1.6 million and plans to reapply for a $750,000 grant. — Darci Rodriguez | The Holyoke Enterprise

Key players in the quest for a local child care facility are pictured July 25 at the future home of the building, from left, Holyoke Community Childcare Initiative board treasurer Tiffany Watson, president Tom Bennett, program coordinator Trisha Herman, board member Krista Doble and secretary Olga Sullivan. Not pictured is vice president Evan Fust. — Darci Rodriguez | The Holyoke Enterprise
-

,Dick Ourada and his 1977 International Harvester cross from Nebraska to Colorado on Highway 6 last week as he and his wife, Carolee, begin a long tractor trip to their home in Fairbanks, Alaska, to raise awareness and funds for Children’s Hospital Colorado Foundation to be earmarked for research. — Courtesy photo

,Dick and Carolee Ourada are almost ready for their nearly 4,000-mile journey to Fairbanks, Alaska, in Aggie, their refurbished 1977 International Harvester 574. They left Holyoke last Wednesday, July 13, and plan to take 60 days to get to Alaska. — Darci Rodriguez | The Holyoke Enterprise

,Dick Ourada modifies the cab he’s adding to his International Harvester at his workshop in Holyoke on July 7. — Darci Rodriguez | The Holyoke Enterprise

At the Central Plains Equipment sendoff July 13 in Holyoke, Dick Ourada, at left, chats with DeeAnn Dubbert about the Tractor Trip for Kids. They were classmates in Grant, Nebraska. — Darci Rodriguez | The Holyoke Enterprise
-

,American flags are one of Audrey Sayles’ favorite things to paint. She made sure there are exactly 50 stars on this mural. — Darci Rodriguez | The Holyoke Enterprise

,Kathy and Carl Schneller’s grain bin mural, painted by Audrey Sayles of Some Girls and a Mural earlier this month, encapsulates everything that is important to them — the Schneller family, their farming business, their Christian faith and their American pride. — Courtesy photo

,It’s a good thing that Audrey Sayles is not afraid of heights as she works on this 30-foot grain bin mural earlier this month. — Darci Rodriguez | The Holyoke Enterprise

Audrey Sayles of Some Girls and a Mural braves the hot sun to paint on Carl and Kathy Schneller’s grain bin east of Holyoke. — Darci Rodriguez | The Holyoke Enterprise
-

,Sporting a lime green swim cap, Jayden Goytia competes at the June 4 swim meet at Holyoke Swimming Pool. — Darci Rodriguez | The Holyoke Enterprise

,Lilli Brinkema comes up for air in the breaststroke. — Darci Rodriguez | The Holyoke Enterprise

Emery Wolf focuses on the task at hand in the backstroke competition. — Darci Rodriguez | The Holyoke Enterprise
-

,From left, Max Kleve, Kenneth Lindholm and Eva Kramer star in Holyoke JR/ SR High School’s production of “Singin’ in the Rain.” — Darci Rodriguez | The Holyoke Enterprise

Max Kleve, at right, holds back overly dramatic Jimena Nuñez from Eva Kramer, at left, and Kenneth Lindholm after an embarrassing accident. — Darci Rodriguez | The Holyoke Enterprise
-
-

,Jessie Owens competes in the sheep show at Phillips County Fair. The Holyoke High School sophomore died in a one-car accident March 21. She will be remembered for her love of animals, children, senior citizens, sports, welding and more. — Courtesy Photo

,Jessie Owens will always be known for her sassy, spunky attitude and great comebacks. — Courtesy Photo

,Jessie Owens grew up on a farm near Holyoke and loved working with her animals. — Courtesy Photo

,Like any good little sister, Jessie Owens, at left, was a shadow of her big sister Rebecca Owens. — Courtesy Photo

Jessie Owens shows her focus, determination and patience while competing with her pig at the Phillips County Fair. — Courtesy Photo
-

,For the first time in Holyoke High School history, the girls basketball team is bringing home a state championship trophy. Emotions are running high as senior team members Lauren Herman, Kristin Vieselmeyer and Elyce Talavera celebrate with a large Dragon crowd after defeating Sanford in the Colorado 2A championship game Saturday evening, March 12, at the Budweiser Events Center in Loveland. — Darci Rodriguez | The Holyoke Enterprise

,Senior Kristin Vieselmeyer attacks the basket during Saturday’s championship game with Sanford. — Darci Rodriguez | The Holyoke Enterprise

,Lauren Herman is up and over a Sanford defender. The senior led the Lady Dragons in scoring during the championship game. — Darci Rodriguez | The Holyoke Enterprise

,Senior Elyce Talavera, at left, works her way around a Sanford player last Saturday in Loveland. — Darci Rodriguez | The Holyoke Enterprise

,Junior Elise Krogmeier dribbles toward the basket during Saturday’s championship game. — Darci Rodriguez | The Holyoke Enterprise

,Audrey Talavera, a Holyoke sophomore, is on fire from behind the arc in the semifinal game against Limon on Friday. — Darci Rodriguez | The Holyoke Enterprise

,In Saturday’s championship with Sanford, junior Grace Roberts pushes inside to attempt a layup. — Darci Rodriguez | The Holyoke Enterprise

,The Lady Dragon bench holds its breath as a shot goes up in the state championship game against Sanford. Pictured from left are coach Kyle Carper, Elise Krogmeier, Hannah Lindholm, Ava Koberstein, Nicole Schlachter, coach John Baumgartner, Fatima Nuñez, Alma Alejandre, Emma Sprague, Anessa Colglazier and Courtlyn Kinnie. — Darci Rodriguez | The Holyoke Enterprise

,A large student section, including Michel Perez, in front, and Jadon Frost, cheers for the girls Saturday at Budweiser Events Center in Loveland. — Darci Rodriguez | The Holyoke Enterprise

The trophy presentation at Budweiser Events Center in Loveland on Saturday, March 12, was cause for celebration for the Holyoke Lady Dragons, who won their first ever state title. Players are pictured from left, Leah Struckmeyer, Elyce Talavera, Grace Roberts, Hannah Lindholm, Audrey Talavera, Ava Koberstein and Kristin Vieselmeyer. — Darci Rodriguez | The Holyoke Enterprise
-

,Greta Parker sits atop Dallas while Rayna Parker and Dawsyn Daniel stand by. Many area people learned to ride on Dallas or sharpened their riding skills with him, and many are feeling the loss after Dallas died in February at age 30. — Photo Courtesy of Brianna Brauer

,Dallas was a horse that impacted the lives of many local riders. — Photo Courtesy of Brianna Brauer

Dawsyn Daniel rides Dallas on a sunny day at Painted Arrow Ranch south of Holyoke. — Photo Courtesy of Brianna Brauer
-
-

,The possible changes made to the sports park area in Holyoke can be seen in this design, which was presented to city council members Feb. 15.

This design depicting the City Park concept for potential changes was presented to Holyoke City Council members by recreation director Victoria Dunker at the Feb. 15 council meeting.
-

,Coltin Houghtelling, in front, celebrates a good round in an entertaining game featured at Melissa Memorial Hospital Foundation’s “Escape to Margaritaville” Legacy Event on Saturday. Other contestants are pictured from left, Shanda Willmon, Jes-c French, Daniel Koch and Luke Garrett. After buying a spot in the game during the live auction, teams of two attempted shots to see who could get the most balls in the baskets. Each round added a new challenge, ending with a large cash prize for the winner. — The Holyoke Enterprise

,A colorful Lane Looka served as emcee for the Legacy Event. He is standing next to a guitar autographed by Jimmy Buffett, one of the featured Margaritaville auction items. — The Holyoke Enterprise

,Nancy Colglazier, Melissa Memorial Hospital Foundation executive director, and husband Harvey kick off the evening’s dancing to Jimmy Buffett’s “Margaritaville.” — The Holyoke Enterprise

,Melissa Memorial Hospital Foundation board member Sarah Bornhoft shows off one of the live auction items last Saturday. — The Holyoke Enterprise

A dancing pink shark makes a surprise appearance at the Legacy Event and shows off the giant pink octopus that serves as the chandelier for the tropical Margaritaville decorations in the Phillips County Event Center. — The Holyoke Enterprise
-

,Heginbotham Library director Kathy Bornhoft is pictured outside the northeast corner of the library, which houses a walled-off secret room. She is standing in a depression that may have been caused by the tunnel that runs from the room to the carriage house on the library grounds. In a building assessment conducted by the State Historical Fund last December, the tunnel was snaked and was discovered to be open except at the carriage house end. — The Holyoke Enterprise

Kathy Bornhoft, director of Heginbotham Library, stands inside the northeast corner of the library. Behind the blank wall at left is a secret room and the beginning of a tunnel that runs to the carriage house. — The Holyoke Enterprise
-
-

,This photo from the June 8, 1995, edition of The Holyoke Enterprise shows the water level of the Frenchman Creek from the Highway 385 bridge on the north side of Holyoke on Sunday, June 4, 1995, after heavy rain in west Phillips County the previous night started the Frenchman flowing. — Enterprise File Photo
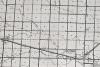
,This 1976 map shows the north and south forks of the Frenchman Creek west of Holyoke. — Source: Phillips County Museum

The Frenchman Creek at Pleasant Valley Bridge is pictured March 10, 1960, after a heavy, wet snowstorm and a sudden melt got the creek flowing. — Source: Charley Triplette
-
-
-

,Carol Fleshman helps one of her alpacas back to its pen. It’s not easy to move alpacas, especially on shearing day. After doing this once every year, however, Fleshman is used to the push and pull of her herd. — The Holyoke Enterprise | Johnson Publications

,Top Knot Shearing Company lays the alpaca down gently before starting on the process where they begin to shear the head of one of Fleshman’s many alpacas. — The Holyoke Enterprise | Johnson Publications

,An alpaca in Fleshman’s herd looks out toward the volunteers after it has been shaved. — The Holyoke Enterprise | Johnson Publications

,Two of Fleshman’s alpacas wait patiently for their turn with the shearer. Fleshman shears her alpacas every summer to help them combat the heat. — The Holyoke Enterprise | Johnson Publications

A crew member of Top Knot Shearing gathers the fleece of an alpaca after it has been sheared. — The Holyoke Enterprise | Johnson Publications
-
-
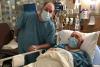
,Kelly Kinnie and Joe Kinnie are pictured from left about a half hour before kidney transplant surgery Jan. 27.

Joe Kinnie and his son Kelly Kinnie are pictured from left at their farm Feb. 23, less than a month after Kelly donated one of his kidneys to Joe in a successful transplant. Joe said farming is where his heart is, and he looks forward to getting back to work there.
-
-

,Community excellence award winners are pictured from left, front row, FCCLA representatives Amy Mackay, Karen Ortner and LorenJo Oberle, Youth of the Year; Alexia Blake of Tuxedo Paws, New Business of the Year; Jan Hewitt, Citizen of the Years; Phillips County Commissioners Terry Hofmeister and Don Lock, Star of the Year; and Joan Owens of Regent Park, Employee of the Year; and back row, Help Holyoke Committee members Tom Bennett, Brenda Brandt, Holly Ferguson and Trisha Herman, Citizen of the Year; and Jake’s Feed representatives Pam Struckmeyer, Briana Worley, Erin Lebsack and Brenda Lebsack with Breck Worley, Business of the Year. — The Holyoke Enterprise | Johnson Publications

Holyoke Chamber of Commerce representatives are pictured from left, Julie Williams, treasurer; Holly Ferguson, executive director; Casey Blake, vice president; Margarita Fierro, secretary; Julie Haake, board member; and McKenna Heldenbrand, board member. Not pictured are Elizabeth Hutches, president, and Michelle Harms, board member. — The Holyoke Enterprise | Johnson Publications
-
-
-
-

,These large wood buttons are displayed on one of 100 panels in Mary Brunken’s button collection at the Phillips County Museum. — The Holyoke Enterprise | Johnson Publications

,Mary Brunken is interviewed by the Enterprise in 1989, the year before she donated her collection of over 12,000 buttons in 12 custom books to the Phillips County Museum.

,Mariann Landes shows off two steamer trunks full of interesting buttons, as well as two button collecting books, which were all passed down from her great-aunt. — The Holyoke Enterprise | Johnson Publications

Out of all the buttons in her collection, this skull is a favorite of Mariann Landes’. — The Holyoke Enterprise | Johnson Publications
-

,Elon Nelson leans against the counter in the room he has reimagined as a 1950’s diner. He has collected many pieces over the years to add to the room’s nostalgic atmosphere, including over 100 clocks. — The Holyoke Enterprise | Johnson Publications

,Elon Nelson’s Pepsi double bubble clocks show the changes made to advertising Pepsi over the years. He has over 100 soda advertising clocks in his collection. — The Holyoke Enterprise | Johnson Publications

,Jerry and Sue Cooper have been collecting Coca-Cola memorabilia for 40 years. Every piece in their collection has a story. — The Holyoke Enterprise | Johnson Publications

This springbok figurine is made entirely of strips of Coca-Cola cans. Sue Cooper purchased it in Botswana while doing missionary work there in 2013. — The Holyoke Enterprise | Johnson Publications
-
-
-

,TISHA & TIFFANY: For Tiffany Watson, age 46 of Holyoke, being a twin means “always having a friend and someone who knows you backwards and forwards.” A downside is that when she goes where identical twin sister Tisha Goodemote lives in Berthoud, everyone thinks Tiffany is Tisha. (Read the full article in print or the e-edition!)

,PIPER & KYRAH: Holyoke twin mom Heather McConachie had her first funny but scary moment when she thought she had mixed up her identical twin girls after taking their hospital bands off. Now, Piper and Kyrah McConachie, age 15, are learning how to drive and making each other laugh so hard they can’t catch their breath. They come from a family with multiple sets of twins on both sides. (Read the full article in print or the e-edition!)

,JOCELYN & JORDAN: Fraternal twin sisters Jocelyn and Jordan Kingman joined a Holyoke family of “J” names when they were born three years ago. On their dad’s side, their great-grandma was a twin, and another great-grandma had twin siblings. Jeanette Kingman, the twins’ mom, said the girls are in tune with each other and never apart. “There isn’t anything they don’t do together,” said Jeanette. (Read the full article in print or the e-edition!)

,KRISTEN & KARINA: Karina (Kramer) Davis, age 32 of Holyoke, thinks it would have been fun to be able to pretend to be her sister Kristen Kramer of Mount Juliet, Tennessee, but the fraternal twins didn’t think they could pull it off. Davis said that being twins put expectations on them that they would have the same likes and dislikes. (Read the full article in print or the e-edition!)

,LINDYN & LAKYN: It’s been a blur, but Holyoke’s Luedke family has made it through the baby and toddler years with 5-year-old fraternal twins Lindyn and Lakyn. When mom Arika went in for her six-week ultrasound, the doctor joked that she was having Irish twins because her son was only 6 months old. “When she did my ultrasound, she heard two heartbeats. I was in shock!” said Arika. (Read the full article in print or the e-edition!)

,JAQUELINE & JESSICA: It’s hard to tell them apart, but Jaqueline and Jessica Mosqueda, age 19 of Holyoke, are actually fraternal twins. They even switched spots for a few days in the fourth grade, and their teacher never found out. The sisters say there are times when they have the same thoughts, and Jaqueline said, “Whenever my sister is nervous, no matter how far apart from each other we are, I can feel her butterflies.” (Read the full article in print or the e-edition!)

,ANDREA & ANGELA: Andrea Kammer, age 39 of Holyoke, admits she still can’t tell who’s who in baby pictures with her identical twin sister, Angela Edwards-Aker of Fort Morgan. In fact, their mom was scared to take the hospital bracelets off until Angela developed a birthmark. And eventually she began dressing the girls in different colors — Andrea in blues and purples and Angela in reds and pinks. (Read the full article in print or the e-edition!)

,DAVE & DAN: In consdering the pros and cons of being a twin, Dave Johnson, age 66 of Holyoke, said he couldn’t think of any cons, but one definite pro is that “you always have someone you can count on.” His fraternal twin, Dan, lives in Kersey and enjoys calf roping, while Dave likes to spend his time golfing. (Read the full article in print or the e-edition!)

,AZUCENA & SUSANA: Azucena Torres, age 42 of Yuma, always laughs when people ask if she and identical twin sister Susana Torres, of El Paso, Texas, are twins, because it’s a pretty obvious “yes!” She said the attention is fun until the silly questions start. “It gives people great pleasure to be able to notice the differences, but yet there are those that we grew up with that still don’t know who is who,” said Azucena. (Read the full article in print or the e-edition!)

BRADY & BRANDI: Fraternal twins Brady Haynes and Brandi (Haynes) Lippert joined uncles Gale and Dale and Garry and Larry in the Haynes family twin tradition when they were born 34 years ago. Brandi, who now lives in Ogallala, Nebraska, said she walked first while her brother Brady, of Sedgwick, was the first to talk. (Read the full article in print or the e-edition!)
-
-
-
-
-
-

,Believe it or not, this is a school assignment. Holyoke teachers are getting creative while providing instruction remotely in response to the coronavirus outbreak. One of first grader Evie Cooper’s recent art assignments was to create a color wheel out of items found at home and send a picture of the finished product to her teacher.

,Using her Chromebook to access Google Classroom, Paxton Gatton-Pollock’s current schooling method bears similarities to a traditional school day despite being conducted at home.

,Brenna Gatton spends one of her modified school days learning to till the garden. While Holyoke teachers provide instruction in their usual subjects online, Jennifer and Scott Cooper are taking advantage of their kids’ extra time at home to teach them about such things as gardening, cars and baking.

,Though Dragon’s Wagon Preschool is no longer in session, Archie Cooper keeps learning at home.

Using headphones is something Hagan Gatton-Pollock and her sisters have found helpful while completing their schooling in close proximity to one another.
-

,The opening of the Londa Wernet Bradford Cancer Center at Melissa Memorial Hospital is celebrated at an open house Wednesday, Oct. 23. Sitting in a comfortable caregiver chair, at left, is Boyce Wernet, Londa’s dad, and at right, in the chemotherapy treatment chair, is Brad Bradford, Londa’s husband. Pictured in back, from left, are Yvonne Wernet, Londa’s stepmom; Patty Wernet, Londa’s sister-in-law; Wes White, MMH chief financial officer; Cathy Harshbarger, MMH chief clinical officer; Ty Wernet, Londa’s brother; Cindy Lock, MMH registered nurse with chemotherapy/biotherapy certification; and Nancy Colglazier, MMH Foundation executive director. The center will soon be open for chemotherapy treatments in Holyoke. — The Holyoke Enterprise | Johnson Publications

New equipment in the Londa Wernet Bradford Cancer Center is kept in a set of three sterile mixing rooms.
-
-

,Librarian Kathy Bornhoft, at left, and staff member Laura Krogmeier admire the chandelier, reportedly from South Africa, that hangs above the circulation desk. A photograph of Will E. Heginbotham can be seen on the wall of what used to be his formal dining room. China cabinets now hold books, and through the doorway patrons can see the original breakfast room with a Dutch mural on the wall. — The Holyoke Enterprise | Johnson Publications

The Craftsman style bungalow at 539 S. Baxter Ave. is one of the most recognizable buildings in Holyoke. It was built for Will E. Heginbotham, a prominent banker, 100 years ago. The mansion, which is pictured in its early days, has served as the local public library since Oct. 5, 1969, and is touted as a community treasure. — Source: Phillips County Museum
-

,This recent display at Coors Field in Denver pays tribute to the 50th-year anniversary of Neil Armstrong and Buzz Aldrin’s walk on the moon. — Photo by Jes-c French

Survey respondents are pictured from left, top row, Tim Bartels, Carolyn Fisher and Gary Krumm; and bottom row, James Scholl, Jeff Tharp and Mary Tomky.
-

,KC Martin is pictured at home with the medal and banner he earned after completing the 140.6-mile Ironman Boulder triathlon June 9. To his right are medals from two other half-length Ironman events that he completed in 2016 and 2017. — The Holyoke Enterprise | Johnson Publications

“That’s why I did it,” said KC Martin, while flipping through photos with his wife, Gina, two days after completing the Boulder Ironman. Martin is pictured with his children — from left, Summer, Teagan and Tayla — after crossing the finish line.
-
-

A photo and a rose honor Cassidy Hale in the memorial garden at the 2018 Donor Dash in Denver. Maggie Busch and Jackie, Tayler and Randy Hale, pictured from left, completed the 5K run/walk to honor Cassidy and other organ and tissue donors, to celebrate the lives of transplant recipients, and to recognize those who continue to wait for a lifesaving transplant.
-
-

,Though she transitioned to assisted living just a few months ago, 99-year-old Mary Allen is making the Carriage House home. — The Holyoke Enterprise | Johnson Publications

In 1940, parents Owen and Eppa Young are pictured with their daughters, from left, Margaret, Wanola, Myrtle, Mary and Stella. Four older half siblings and three brothers who had died by that time make up Mary Allen’s large family.
-

,Inside the sanctuary of the former First Baptist Church building, Matt Cole has established his collection of golf memorabilia, including a nine-hole indoor putting green. — The Holyoke Enterprise | Johnson Publications

A tee marker from hole six on the Carnoustie Golf Links in Carnoustie, Scotland, sits among the items in golfer Matt Cole’s collection. Behind the marker is a baptismal font formerly used by First Baptist Church and a wall of banners featuring famous golfers. — The Holyoke Enterprise | Johnson Publications
-

,Adding $7,800 to the evening’s fundraising total, Spinning on the Bayou was a new game at this year’s Legacy Event. Six chances to play the game were auctioned off, and the six winning bidders sent designated “spinners” to the front of the room to play. Cheered on by his team, Brady Dirks spins the wheel, while Melissa Memorial Hospital Foundation board members Jessie Ruiz Jr., Tiffany Weber and Brady Ring, pictured from left, wait to see his fate. — The Holyoke Enterprise | Johnson Publications

Southern Charm costumes at the Feb. 9 MMHF Legacy Event featured such things as hoop skirts, gunny sack dresses, cowboy hats and Confederate soldier uniforms and represented southern locales including New Orleans and Mexico, just to name a few. Enjoying the social hour in costume are Chad Huffman, Jennifer Cano, Heather Huffman and Jason Greenman, pictured from left. — The Holyoke Enterprise | Johnson Publications
-

,Sam Distefano, playing the role of a rescuer, signals a team on the shore of the Lions Club Fishin’ Hole, while Dustin Carrick, playing the victim, lies prone on a rescue sled. The simulated rescue was conducted during a Jan. 11-13 training for Holyoke Volunteer Fire Department and other nearby rescue agencies. — The Holyoke Enterprise | Johnson Publications

Scott Korte of Holyoke Volunteer Fire Department and rescue instructor Dave Martin are pictured from left, as Martin gives encouragement to Korte, who is attempting to pull himself out of the frozen-over Lions Club Fishin’ Hole. — The Holyoke Enterprise | Johnson Publications
-

,Shayla Hecht, pictured at center, gets her game face on and competes as a blocker for the Flat Rock Roller Derby team based in North Platte, Nebraska.

While at a photo shoot for the Flat Rock Roller Derby Team, Molly Weils, at right, shows off the traditional roller skates used in the fast-paced contact sport.
-

,Ground has been broken and the footprint is starting to take shape for the new Phillips County Pavilion and Education Center at the fairgrounds. Pictured from left, county commissioners Harlan Stern, Don Lock and Joe Kinnie are at the work site, where project manager Matt Brasby said progress on the foundation is ahead of schedule. Skarco Design of Burlington has been contracted for the concrete foundation, and Maverick Steel of Byers has been contracted for both the building materials and its erection. The pavilion is expected to be finished in time for the the 2018 Phillips County Fair. — The Holyoke Enterprise | Johnson Publications

,Mike Frazier measures hail that’s 3 inches in diameter — about the size of a baseball — at his home northeast of Amherst.

Holyoke EMS director Brady RIng, pictured at center, addresses his junior EMT class at their final lesson May 23, which included setting up an on-scene landing zone and touring an AirLife helicopter. — The Holyoke Enterprise | Johnson Publications
-

It may not look like much to someone driving down a stretch of county road, but this mailbox has been standing throughout Bob Brandt’s entire mail carrying career — and that’s no small feat for a country box. The house that it accompanies has been home to three generations of a single family, and families with deep roots in Phillips County are commonplace on his route.
-

,Holyoke’s Cap Holtzman, pictured at left, does some sparring during World War II. It was common at the time for boxers to keep up with the hobby during their time in the service.

Among old posters collected by the descendants of local 1930s boxers, this April 5, 1932, event is the earliest Holyoke Stadium fight represented.
-
-

,Horses and buggies drop off customers at the mercantile at 105 S. Interocean Ave. in Holyoke. A business partnership in the early 1900s called LeBlanc and Scheunemann was profitable and resulted in this brand-new brick building constructed in 1907.

While its front exterior and flashy neon sign — both from the 1950s — are different from the original structure, Scheunemann’s Department Store remains a business icon in Holyoke in 2018. — Johnson Publications
-
-
-
-

,Kaitlyn Thomas says her last goodbyes to her goat, Zach, before the junior livestock sale at the Phillips County Fair on July 28. The sale, which was held in the new Phillips County Pavilion and Education Center, was an emotional day for many in 4-H and FFA. — Johnson Publications

Cousins Brandon and Austin Durbin are pictured from left with Austin’s goat, Ty, before the junior livestock sale at the Phillips County Fair. — Johnson Publications
-

A new administrative team at Holyoke School District Re-1J is already at work to prepare for the start of the 2018-19 school year. Administrators are pictured from left, Shane Walkinshaw, JR/SR high principal; Kyle Stumpf, superintendent; and Andrea Kammer, elementary principal. — Johnson Publications
-
-

,Tayla Martin takes first place in the 100-yard freestyle, setting a new record for 11-12-year-old girls. — Johnson Publications

Holyoke Swim Club is well-represented in the 15-18-year-old girls 100-yard breaststroke. Pictured from left, Anna Jelden placed fourth, Traeli Hutches third, Rylee Schneller first and Regan Van Overbeke second. — Johnson Publications
-
-

,Highline Electric Association crews are out in full force Saturday, April 14, to rebuild power lines and restore power to rural residents. Matt Miller, at left, and Eric Luedke do work from the buckets south of Fleming as warmer temps quickly melt the snow.

Stacy Fulscher’s cattle weather the storm that blew into northeast Colorado Friday afternoon, April 13.
-
-

,Carl and Kathy Schneller are anything but weary travelers. The Holyoke couple has traveled to roughly 85 countries and can’t wait to plan their next vacation. Here, they are pictured at Machu Picchu, the lost city of the Incas, in Peru.

Carl and Kathy Schneller strike a pose at the Pyramids of Giza and the Great Sphinx near Cairo, Egypt.
-

,In fierce Bible quizzing competition, the first person to stand up gets to answer the question. A typical quiz looks much like this, with quizzers attentively listening to the questions and physically ready to jump up as soon as they’re ready to answer. The team pictured competed in the Youth Challenge 2018 Jamaican Bible quizzing tournament earlier this month. — Photo by Emily Malmkar | EMDesign

,Frances Patterson, seated at left, works with the team of students from Coronaldi School in Montego Bay, Jamaica, while students from last year’s Bible quizzing team look on. Formerly of Dailey, Patterson now lives in Omaha, Nebraska, and has traveled to Jamaica with Youth Challenge nine times. — Photo by Emily Malmkar | EMDesign

This particular photo was taken on a February 2014 trip to Jamaica, but such scenes are common, as Rick Cleaver, pictured at right, has served on close to 60 missions trips to Montego Bay with Youth Challenge.
-
-

Community Excellence Award winners at this year’s Holyoke Chamber of Commerce Gala are pictured from left, Citizen of the Year Casey Blake; STAR of the Year Star 92.3’s Marc Maelzer and Michelle Harms; Employee of the Year Earl Downing; Youth of the Year Shianne Willmon and Josie Herman; and Business of the Year Cobblestone Inn & Suites’ Sue Razo, Scott Murray, Amber Salas, Jorge Salas, Carol Kumm and Bailey Hamaker. Not pictured is Youth of the Year Emily Jelden. — Johnson Publications
-

,Holyoke third-graders get their first glimpse of the solar eclipse Monday, Aug. 21. Students are pictured from left, front row, Alexx Mateo Zazo and Claire Hubbard; second row, Ricardo Goytia, Elia Wear and Sophie Rahe; and back row, Peyton Adams, Maelynn Frost, Daniela Castillo Marquez, Jackie Santiesteban and Roxy Santos. — Johnson Publications file photo

,This scorched semitruck lies on the Phillips-Logan county line, a fitting representation of the violent power of wildfires. No lives were lost in the March 6-8 incident, but extensive property damage covering 50 square miles became evident as the smoke cleared. — Johnson Publications file photo

Holyoke High School boys basketball team, led by head coach Scott Dille, brought home the championship trophy from the 2A state tournament in Loveland, giving them bragging rights alongside the two other HHS boys teams who won the title in 2010 and 1984. Team members get their hands on the coveted trophy after defeating Sedgwick County 44-41 Saturday, March 11, in front of a massive Holyoke crowd. Emotions are running high for team members, pictured from left, in front, Austin Herman, Tyler Camblin, Arturo Dominguez and Brendan Mayden; and in back, Alex Strauss, Slaten Burris, M.J. Taylor, Wyatt McCallum and Anthony Beltran. — Johnson Publications file photo
-
-
-
-
-

,Dragon Blake Mosenteen gains ground for the Dragons behind the blocking effort of Seth Watson in Holyoke’s 12-8 win over Bridgeport Friday, Sept. 1. Mosenteen led the HHS rushing stats with 140 yards in 22 carries and scored a touchdown in the season opener. — Johnson Publications

Dragon Trent Huffman (63) pulls down Bridgeport quarterback Drake DeMasters for a nice sack midway through the first quarter of Friday’s football opener. — Johnson Publications
-

,In addition to helping line up the Phillips County Fair parade for many years, the Holyoke Lions Club often gets in on the candy-throwing action themselves. In the 2012 parade, Don Beckius drives a golf cart decorated with lions, offering a smile and a wave to everyone lining the street. — Johnson Publications file photo

Bingo nights from days gone by were made possible by Lions Club members, pictured from left, Don Beckius, Gene Hinck, Jean Brown, Larry Stein, Leroy Smith and Rich Brown. Bingo is not only fun to play but also raises money for a college scholarship.
-

,Cesar Favela, in front, and Paige Marlow, Holyoke second-graders, gaze at the highly-anticipated solar eclipse Monday, Aug. 21. — Johnson Publications

Holyoke third-graders get their first glimpse of the solar eclipse Monday, Aug. 21. Students are pictured from left, front row, Alexx Mateo Zazo and Claire Hubbard; second row, Ricardo Goytia, Elia Wear and Sophie Rahe; and back row, Peyton Adams, Maelynn Frost, Daniela Castillo Marquez, Jackie Santiesteban and Roxy Santos. — Johnson Publications
-

Mothers sending their children to the next step in school hold their little students’ baby pictures close. Pictured clockwise from top left, Ashley Clayton and son Levi, who is entering kindergarten; Letty Penzing and daughter Juana, who will start at Purdue University; Tracy Steggs and son Bo, who is entering kindergarten; and Brenda Krueger and daughter Tara, who will start at Colorado State University. — Johnson Publications
-
-

,This photo of two WWI soldiers in uniform is part of the Phillips County Museum collection, where actual uniforms are also on display. The man pictured at left is identified as Lauritz Peterson. (Source: Phillips County Museum)

This is only a portion of a giant panoramic photo housed at the Phillips County Museum. In its entirety, the photo is a sight worth seeing in person. It is one of the many items donated to the museum by Leslie Taylor, who served in World War I and later lived in Haxtun. According to the museum, the photo is probably of the 35th Infantry Regiment at Nogales, Arizona, in 1918. (Source: Phillips County Museum)
-
-
-
-

,See that small line running across the photo? Anchored between two trees about 6 feet off the ground, that slackline is Dylon Lousberg’s ticket to international trickline recognition. —Johnson Publications photo

The average trickline measures just 2 inches across, but Dylon Lousberg has no problem maintaining balance as he bounces from his back to his feet to his chest. —Johnson Publications photo
-
Facebook Feed
Holyoke Enterprise
970-854-2811 (Phone)
130 N Interocean Ave
PO Box 297
Holyoke CO 80734